Five reasons to choose StemRNA 3rd Gen Reprogramming Technology
RNA Reprogramming is used globally for the generation of integration-free induced pluripotent stem cells (iPSCs) by researchers in biotech, academia, research hospitals and government agencies. This reprogramming method is gaining more and more interest and core labs and biotech companies focusing on regenerative medicine and GMP compatibility are adopting this technology and make it their standard.
REPROCELL’s latest evolution in the Stemgent RNA reprogramming kit series combines a unique non-modified RNA and microRNA technology to generate induced pluripotent stem cells (iPSCs). This novel StemRNA 3rd Gen Reprogramming Kit provides stem cell researchers with a new level of simplicity, versatility, and time saving, enabling cellular reprogramming of human fibroblasts, cells derived from blood and now urine even on difficult to reprogram patient samples.
REPROCELL offers a complete portfolio of services for the reprogramming and differentiation of high-quality iPSCs.
StemRNA 3rd Generation Reprogramming Technology
Stemgent® StemRNA™ 3rd Gen reprogramming technology is the most rapid, robust, and clinically- relevant integration-free method on the market. We have listed five scientific reasons that this reprogramming technology is so popular with stem cell scientists across the globe.
1. Requires No Screening of iPSCs
Non-viral, non-DNA, non-integrating cellular RNA reprogramming allows for the generation of clinically-relevant iPSC lines without the need to screen clones for vector retention (footprint-free) — this saves you time and money! [See data in ref. 1]
2. Generates High Quality iPSCs
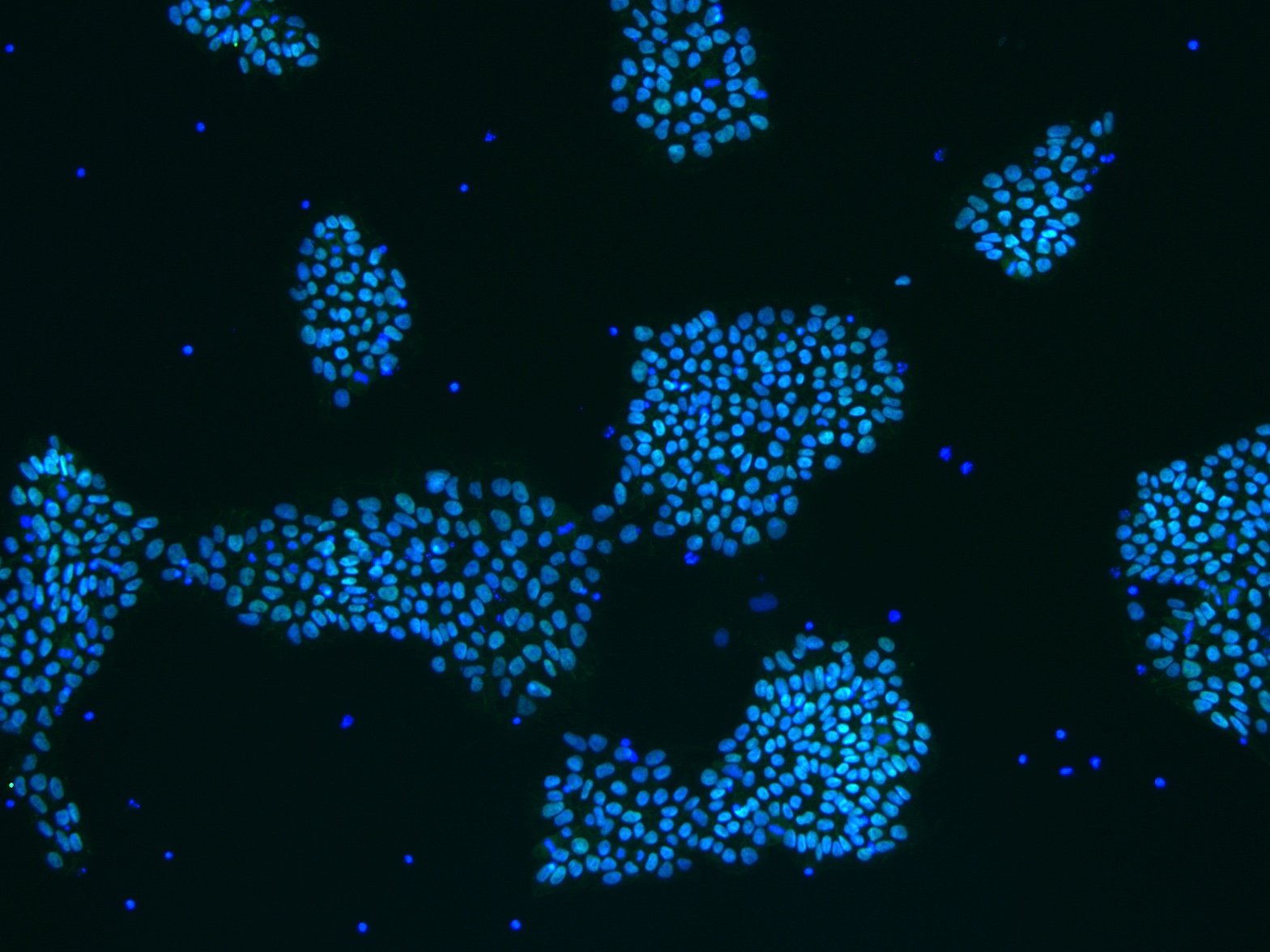
StemRNA-reprogrammed iPSCs are robust in culture and display very low clone-to-clone variability with normal karyology. In figure 1, reprogramming progression and iPSC characterization are shown on fibroblasts, UPCs and EPCs as performed by our scientist. The left figure illustrates the progressive speed and robustness of the reprogramming process using the StemRNA 3rd Gen technology. The right figure shows, pluripotency validation of generated iPSCs by marker expression and by in vitro and in vivo differentiation assays.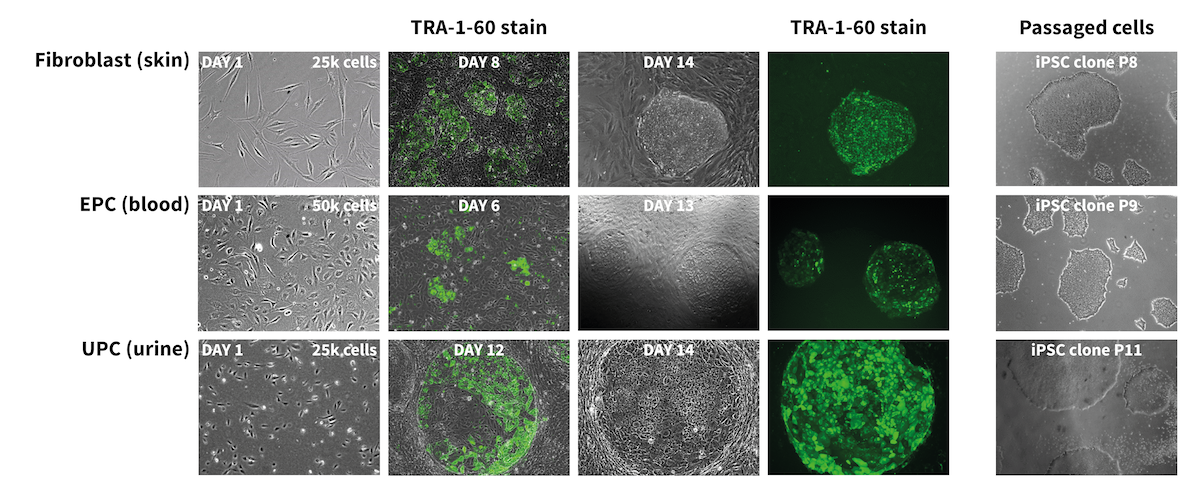
Figure 1: StemRNA 3rd Gen reprogramming technology provides high-quality, fully pluripotent iPSCs starting from skin (fibroblasts), blood, or urine
3. Yields Robust and Consistent Results
The combination of a unique microRNA cocktail with the use of non-modified RNAs increases the overall efficiency (Figure 2) and enables success for refractory or difficult-to-reprogram patient-derived samples across multiple different somatic cell types (Figure 1).
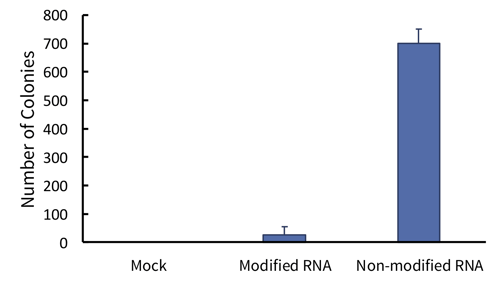
Figure 2: Non-modified RNA leads to a greatly increased efficiency or iPSC generation. Human fibroblasts were either mock transfected, transfected with traditional modified RNA, or transfected with StemRNA 3rd Gen non-modified RNA. Colonies were stained for alkaline phosphatase and counted on day 19 after the start of transfections.
4. Uses Safe and Clinically Relevant Methods
The non-modified mRNA in the StemRNA 3rd Gen method yields high reprogramming efficiencies of between 1-4% of primary cells, depending on the cell type, with iPSCs ready to be clonally expanded by day 10-14.
 Figure 3: Time line for fibroblast reprogramming with StemRNA 3rd Gen technology
Figure 3: Time line for fibroblast reprogramming with StemRNA 3rd Gen technology
5. Safe and clinically compatible
The non-modified RNAs of our StemRNA 3rd Gen technology are synthesized using a GMP-compatible manufacturing process. The reprogramming protocol and the iPSC maintenance medium (NutriStem® hPSC XF Medium) are chemically defined, xeno-free and serum-free. NutriStem is produced under cGMP compliance, with an FDA drug master file available.

Figure 4: REPROCELL has reprogramming technical experts and stem cell laboratories on three continents

REPROCELL offers a complete portfolio of services for the reprogramming and differentiation of high-quality iPSCs.
RNA Reprogramming Services at REPROCELL
REPROCELL offers StemRNA-3rd Gen Reprogramming Kits and custom iPSC production services to clients in industry and academia with over 15 years’ experience as leaders in stem cell research.
Our custom services provide iPSCs and differentiated functional cell types suitable for your own use in the commercialization of services and products as well as research purposes. The 3rd generation RNA technology is faster, more stable and footprint-free, allowing to deliver iPSCs in around half the time of its competitors.
How does our StemRNA 3rd Generation Reprogramming Kit compare with others?
|
Comparison of Non-Integrative Reprogramming Kits |
||||
| Reprogramming technology | Sendai ThermoFisher |
Episome ThermoFisher |
StemRNA 1st & 2nd Gen REPROCELL (Stemgent2) |
StemRNA 3rd Gen REPROCELL (Stemgent2) |
| Non-integrative | ✓ | ? | ✓ | ✓ |
| No vector retention | × | × | ✓ | ✓ |
| DNA-free/ Virus-free | × | × | ✓ | ✓ |
| No screening required | × | × | ✓ | ✓ |
| Normal karyology and stability of iPSC | ++3 | +3 | +++3 | +++ |
| Reprogramming efficiency | ++3 0.08% |
+3 0.01% |
++3 1% |
++ 2-4% |
| Reprograms refractory patient lines | × | × | ✓ | ✓ |
| Time to usable iPSCs* | 8-10 weeks (Passage 10+) |
10-12 weeks (Passage 10+) |
5-6 weeks (Passage 4-5) |
4 weeks (Passage 4-5) |
*Includes time needed for reprogramming, colony isolation/expansion, and vector clearance (if applicable)
References
- Schlaeger et al. A comparison of non-integrating reprogramming methods. Nature Biotechnology 33:58 (2015)
- Poleganov et al. Efficient reprogramming of human fibroblasts and blood-derived endothelial progenitor cells using non modified RNA for reprogramming and immune evasion. Human Gene Therapy 26:751 (2015)
- Liu et al. Comprehensive characterization of distinct states of human naive pluripotency generated by reprogramming. Nature Methods 14:1055 (2017)
Editors note: This article was first published in March 2018 and has since been updated for accuracy and clarity.

Author
Anca Haralambie, MSc
Subscribe to receive updates from REPROCELL
Tagged
Stem cell and drug discovery scientists around the world are using REPROCELL’s services and products in their preclinical and clinical research.